Happy Spring everyone, it is finally spring and the long winter is over. Of course on the first day of spring here in D.C. we got some snow. Go figure. I’ve had a lot going on lately with home life and work life and sort of let this blog go to seed for more than a month. I am back and have about 8 posts in the queue but I haven’t scheduled them yet…they are not ALL saisons…although several are.
I think it is about time for another one of these science posts. This blog isn’t just about hoppy saisons, although you can read all about those in the recipe posts. This blog is suppose to be about brewing and beer science (at least a portion of it). You can’t have a blog about brewing science without doing a post about the physiology of Lactobacillus. This is part of a slow motion suite of posts about the physiology of beer and fermentation, the first of which covered Saccharomyces cerevisiae, or brewer’s yeast. It is part of a larger goal of making a bunch of posts about the microbiology of beer. Since I covered yeast in the last one, I decided to move into the world of prokaryotes (bacteria) and cover one of the more common groups of bacteria that are used in fermentation. I received a lot of great feedback through email and comments on the last post, I hope people will do the same for this one to improve the quality of this blog and specifically these posts overall. So, Lactobacillus….
What is Lactobacillus?
Lactobacillus is a genus that covers a group of bacteria responsible for the production of lactic acid through the fermentation of sugars. In the language of microbiologists, they are Gram-positive (no outer membrane), rod shaped, facultative anaerobes (can use oxygen some of the time but grow fine without it) and represent a significant portion of the “lactic acid bacteria” (sometimes referred to as LAB) which is an order of bacteria that include several dozen genus and thousands of species. There are currently more than 150 different species of Lactobacillus identified (not to mention subspecies and the ones people still argue about). Lactobacilli are bacteria (prokaryotic) as opposed to Saccharomyces and Brettanomyces which are eukaryotes. Bacteria and eukaryotes are fundamentally different. Bacteria are generally much much smaller, grow much faster, and have simplified (but not simple, see below) physiology. Eukaryotes form subcompartments within their cells to specialize in a particular process, the most obvious being a nucleus to store the DNA. Eukaryotes have nuclei, prokaryotes do not.
Lactobacillus is one of the bacteria (along with Pediococcus as well as a few others) responsible for all those wonderful sour beers you taste. Berlinerweiss, Gose, lambic and whatever Americans are calling their sour beers these days, are all made with different species of Lactobacillus. The popular “funky” yeast Brettanomyces is not the organism that sours a beer (typically), this is a common misconception that is fortunately going away. Lactobacillus species aren’t only responsible for souring beer but actually are responsible for the sourness of many amazing foods (basically all the best foods) such as sourdough bread, kimchi, saurkraut, and yogurt (and hundreds of other dishes). As a homebrewer, we can purchase a few different species / strains of Lactobacillus that are supposedly pure cultures. These species include (but perhaps not limited to) L. buchneri, L. brevis, L. delbrueckii, L. rhamnosus and L. plantarum. Lactobacillus species all share a few physiological characteristics, primarily that 50% of the carbon produced goes to lactic acid (or lactate, for the purposes of this blog those terms are interchangeable). While several of the other metabolites will be covered below, some Lactobacillus species can also produce ethanol, acetic acid (vinegar), succinate, and formate.
Different Species of Lactobacillus
There are currently more than 150 different species of Lactobacillus. This is in contrast to Saccharomyces with about 20 species (some being debated still) and Brettanomyces with less than 10 species (also still subject to debate). While there are 150 annotated species, there are surely hundreds if not thousands more yet identified or classified. This huge number is, at least in part, due to the primitive way species were originally classified as Lactobacillus. “Is it a rod? Is it Gram positive? Does it make lactate? Then it is Lactobacillus.” That is a pretty accurate depiction of how organisms were established as a member of this genus. In the past 20 years, there has been significant shuffling of dozens of species into new a genus or even a family of bacteria. The concept of species at the molecular level is more fluid than those of us in the macro-world would sometimes like. Two different strains of the same species might share only 80 or 90% of their DNA, to put that in perspective humans and cats share 90% of their DNA. A species of Pediococcus was recently reclassified as a Lactobacillus based on some DNA sequencing. A few species have been combined into one as well. This effort is ongoing, which makes it hard to really put down a solid number of species. Instead of tackling that pile and wade through the phylogeny of an ever changing landscape of species and strains, I’ll highlight a few of the better characterized ones or ones I’ve seen people use in the past for beer or food production and speak in great detail about them. I already singled out a few different species common to beer production, at least at the homebrew scale: L. buchneri, L. brevis, L. delbrueckii (various subspecies), and L. plantarum. As these posts generally run long as it is, I will keep the focus on a dozen or so species, and will summarize as much information as possible into tables so people can take what information they want for many organisms.
The physiology of Lactobacillus Species – Primary Fermentation Products and Carbon Utilization

The physiology of Lactobacillus is pretty diverse, given the number of species this probably isn’t a surprise. The first thing we have to mention is that the end product of fermentation by this genus will vary based on what organism is doing the fermentation. There are “heterofermentative” and “homofermentative” species (and a third designation that is roughly between the two). Homo-fermentation is easy to grasp. In those cases, the majority of the carbon (>90%) ends up as lactic acid (lactate). Lactic acid is three carbons and (most) sugars, such as glucose and fructose, are six carbons. For every sugar metabolized, two lactate molecules are produced. That is homo-fermentation (sometimes called homo-lactate fermentation).
Heterofermentation is what you probably suspect, the cells make more than just lactate as a final product. Lactate is still the major product (>50% of the carbon goes to lactate) but in a heterofermentative Lactobacillus, you will also get ethanol and some acetic acid. Below is a table that summarizes the relative amounts of lactic acid, acetic acid, and ethanol made by 10 different species of Lactobacillus. Saccharomyces cerevisiae is included in the table for comparison. Even though Lactobacillus can make ethanol, it usually only makes 10-20% the total that S. cerevisiae can crank out in the same time under similar conditions. Again, for all 10 strains, the majority of the carbon is directed to lactic acid. The acetic acid levels are above the flavor threshold in some of the species, so it will be noticeable, however the concentration of the lactic acid is 10× higher than the acetic acid in all of these species and is the dominant flavor component as well as the primary cause of the drop in pH of the fermentation.
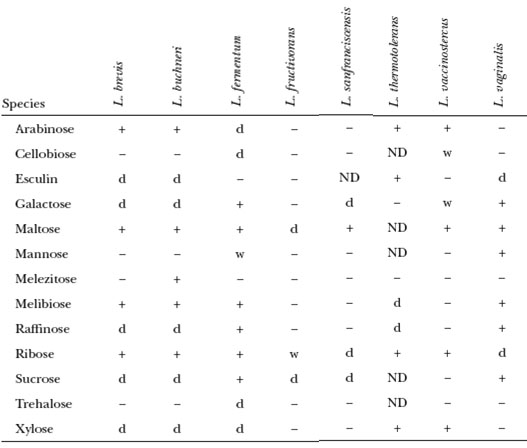
 One question that comes up frequently when discussing Lactobacilli with microbiologists or sour beer enthusiasts is about carbon utilization. For decades, bacteria were classified based on what sugars they could ferment and what those fermentation products were ultimately. To determine this, you simply attempt to grow the species in a minimal media with only one carbon source, glucose for example. I adapted the carbon utilization tables from a version of the Bergey’s Manual below for anyone that is curious about what species can ferment what sugars.
One question that comes up frequently when discussing Lactobacilli with microbiologists or sour beer enthusiasts is about carbon utilization. For decades, bacteria were classified based on what sugars they could ferment and what those fermentation products were ultimately. To determine this, you simply attempt to grow the species in a minimal media with only one carbon source, glucose for example. I adapted the carbon utilization tables from a version of the Bergey’s Manual below for anyone that is curious about what species can ferment what sugars.
That is a lot of information. It comes up a lot so I thought I would attempt to summarize some of the more relevant strains but again not all 150 by adapting those tables. To make it more relevant, maybe we can a few salient examples out of those tables for a moment. One of my favorite species to work with the Lactobacillus plantarum. It sours fast, it more on the homofermentative spectrum of things (no ethanol production) and according to those tables can ferment just about any sugar it encounters. This will make L. plantarum a high attenuating strain and could potentially sour a wort using sugars not available to other organisms in the fermentation. In the last table, L. buchneri and L. brevis can both ferment a variety of sugars but not cellobiose or trehalose. Lactobacillus delbrueckii subsp lactis can consume trehalose which is an off flavor associated with yeast autolysis as trehalose is released when Saccharomyces cells lyse. L. delbrueckii lactis would be great for fermenting a variety of sugars that may be present in wort or other food products especially in conjunction with other species such as L. plantarum.

Secondary Metabolism
Now we are to my favorite part of these posts, huge tables of flavor or aroma active chemicals made as secondary reactions to the central metabolism. Even though some of these species are classified as “homolactic fermenters”, lactic acid far from the only product of their metabolism. Both the homo and heterofermentative species of Lactobacillus produce a variety of secondary metabolites that can have flavor and/or aroma implications. Below is a summary of various species and the amount of secondary terminal metabolites they may produce as a result of normal physiology. I had a difficult time estimating the concentrations of the different compounds for each of the organisms since I am covering so many different species. I had to use multiple sources to assemble these tables, each paper did the experiments differently and the studies spanned more than a few decades. I chose to use the “+” and “-” designation for species in the table but that does not express the relative amounts of each compound. Obviously, the greater the concentration, the more obvious the flavor. For example, acetylaldehyde production by the homofermentative strains, L. delbrueckii makes the least amount of that compound under similar growth conditions while L. acidophilus makes 50 times the amount under similar conditions. I will point out the large differences when I can. In the table, when there is a large difference in the overall amount, I noted it as “+++” compared to “+”. The “+/-” notation means that the range reported includes “zero” as a measurement. This should be interpreted as “trace” amounts of that compound. An amount that might be detected but not necessarily by everyone.

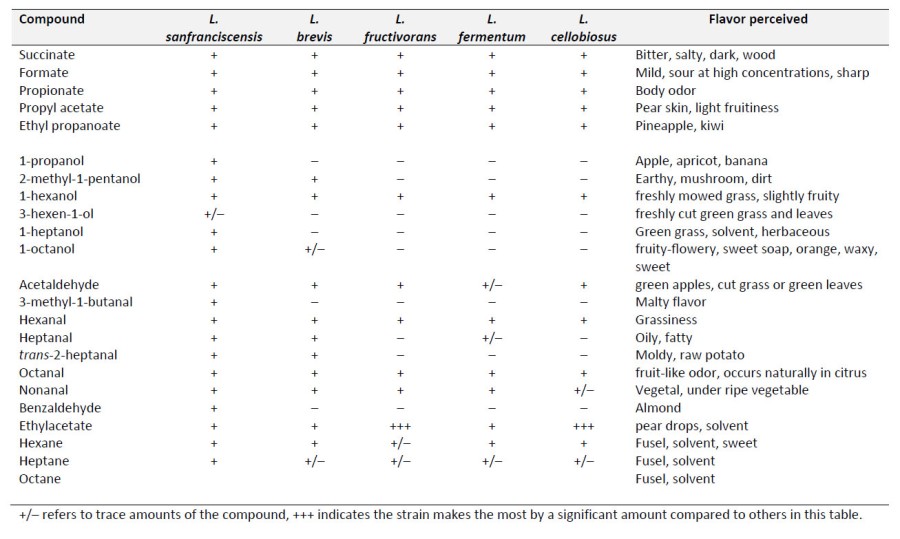
One of the more interesting trends that people might care about is that none of the heterofermentative species produce diacetyl at any detectable quantity. The homofermentative species Lactobacillus alimentarius produces the most and Lactobacillus delbrueckii makes only trace amounts, although right on the edge of human detection. The difference between the two is about 20-fold. L. sanfranciscensis is an interesting species. If you can’t tell the by the name it was isolated in San Francisco from sour dough bread. It makes the highest measured concentrations of most of those compounds and makes the most diverse collection (it makes everything on the table except sometimes not 3-hexen-1-ol). I thought about why this might be the case compared to other strains and whether or not this would make it better or worse for use in beer production. While I haven’t conducted an experiment myself….I think that species would be horrible to make beer. While the flavors of sour dough can be wonderful and complex, many of those compounds would evaporate at the temperatures used to make bread (especially the long chain alcohols). I have never done it (but now sort of want to) but it would be fun to do a 100% L. sanfranciscensis fermentation and see if you can pick up the fusel alcohols.

Phenolics and Lactobacillus plantarum
For some reason, there is a lot written about Lactobaillus plantarum and food fermentation. I guess it is the one of the more popular ones to study. It seems that L. plantarum has several enzymes that allow it to metabolize a huge variety of compounds from plants such as bran, olives, cabbage, various beans and squashes….resulting in a huge pantheon of compounds. I decided ultimately not to cover that in this post but perhaps write something specific about that species since it is so widely covered in the literature.

The compounds in the above figure are found in various plants…and some of these compounds can inhibit the growth of Lactobacillus. These are of note because L. plantarum can metabolize them to other compounds that are less toxic…sometimes resulting in a new compound that has an aroma or taste property a human can detect. This post is already long enough so think of it as part 1 of 2.
Summary and Final Thoughts
Lactobacilli are the 800 pound gorilla in the room when talking about sour foods and beverages. Lactobacilli have a huge role in food microbiology (I used them in my chile pepper sauce experiment) and are generally seen as healthy or at least normal in human diet. I do not want to overstate the “probiotic” argument some people make for these organisms as from a scientific point-of-view those arguments are mostly specious. I can post something separate about that if I have time. I believable that we co-evolved to live together and benefit from each other. Afterall, the organism that can eat and thrive on fresh food AND “spoiled” food has a greater stock of food to try. I love foods and beverages made with Lactobacillus species….I’m currently making sour dough bread using spent grain flower and Lactobacillus brevis (post on that coming up). There has to be some thoughtful experiments and follow-up on the different species as their individual flavor contributions are going to be significantly different. I am planning a preliminary experiment to this end soon, see below.
Personally, I think Lactobacillus plantarum is the most interesting from my preliminary tests with taste and after literature searches for a homofermentative species. I think Lactobacillus brevis is probably my go to species for heterofermentative. A more definitive experiment underway already.
Upcoming Posts
I took most of the month of March off due to life and other things. I have a baby coming soon as well so there may be a break around there as well. There are a few Lactobacillus related articles coming up in the near future. I did a pH experiment where I measured the drop in pH over several days of four different species at different temperatures. The results were pretty interesting. I’m starting a post like this one about Pediococcus, I don’t know how long that will take to get up though, these posts take a while. I’d like to do more practical experiments as well. I am planning on doing small scale fermentations of simple wort with different pure cultures of Lactobacillus and have people taste the result. See if these side products or secondary metabolites really do play a role in the final product. Finally, I did an experiment recently where I spiked a beer with the two isomers of lactic acid (D and L) to see if a small tasting panel could tell the difference between the two. There is definitely a taste difference. I am currently researching ways to repeat this experiment in a more controlled way, so that might be a write up in the future. I hope you are enjoying these diversions into microbiology. I like writing them when I have the time. I’ll be updating the blog more frequently now.

“I never had problems with my fellow scientists. Scientists are a friendly, atheistic, hard-working, beer-drinking lot whose minds are preoccupied with sex, chess and baseball when they are not preoccupied with science.” ― Yann Martel



Thanks so much for this, Matt!
Matt, this post is so unbelievably helpful. As a part of my NHC presentation on Acid beers I’d like to include some of this information. I’ll send you an email with some follow-up questions as well.
I’m also currently running an experiment with 7-9 available lacto strains.
Thanks for this.
shoot me a message through FB or matt.humbard gmail
Great post on lacto. Have been brewing in my micro class last few years. This summer chemist and i are using his new LC/MS to look at acid products from Enterobacteriaceae for use in class next year. Might switch to Lacto strains as I am starting sour brewing at home.
So you do small molecule work then? want to collaborate? 😀
Yes we are working out method to determine fermentation acid products. We were working on hop acids at first (and still interested) BUT we really do need to develop an assay we can use in student labsnext winter term. Lacto bugs fits the bill nicely.
We would be glad to do collab. Might be easier to email me direct @ roy.ventullo@wartburg.edu.
We have the HPLC method for acids working and can see succinate, acetic, lactic. Need to work on GC method for ethanol as we do not have HPLC detector for ethanol.
Matt, this is a great write-up, and I’m looking forward to the follow up posts, as well as the results of your sensory tests on various species of lactobacillus. If you need any brewers to help with the experiment, I would be happy to volunteer. Jeff, the same goes for you. I owe you a visit, and enjoyed the group testing with the Great Brett Experiment.
I’ve been making sour meads and exploring LAB for souring. It’s a whole different animal doing fermentation with honey with SAC and LAB.
I’ve sent in some samples to ETS labs with really tasty LAB fermentations that started with natural LAB in the honey (at first by accident). Award winning in competitions. They were identified as Casei/Paracasei/Mali/Nagalli (evidently closely related?)
My temps are low 60’s in my winery, if not cooler.
Today I made up a couple 50 liter kegs of starter with Omega LAB blend. I understand it will rock out at lower temps.
I think that the LAB slows down the SAC fermentation which allows the LAB time to work it’s magic to make lactic before the SAC makes too much alcohol and puts it out of commision. Tricky business. Challenging journey.